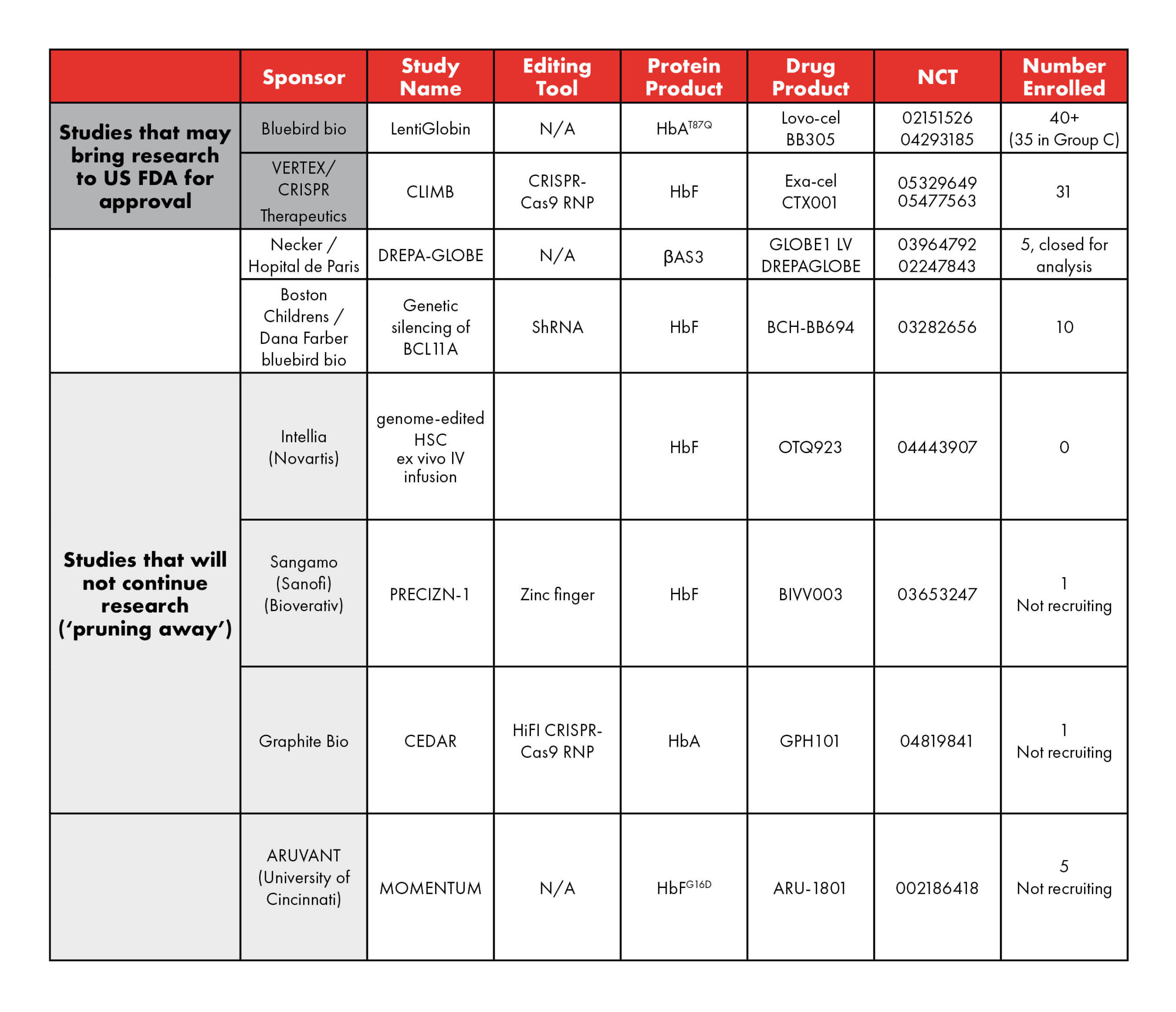
MARAC Statement: Health Insurance Coverage for Hematopoietic Stem Cell Transplant for Sickle Cell Disease from HLA-matched Sibling Donor (MSD HCT)
Sept. 16, 2023
BACKGROUND
Hematopoietic stem cell transplant for sickle cell disease from HLA-matched sibling donor (MSD) after myeloablative conditioning has been proven as curative therapy for sickle cell disease for over 25 years. It is no longer considered an experimental procedure. Over 2,000 transplants have been performed successfully across the world from HLA-matched sibling donors. Pooled Overall Survival rates for children and adults are high at 97% and 98%, respectively (meta-analysis by Iqbal 2021). The outcomes in sickle cell disease are much better for younger ages, but outcomes can still be good for selected adults. MSD HCT is expensive, but multiple analyses have shown that long-term health care costs go down, quality of life improves and organ damage is usually slowed or stopped. This means that MSD HCT is very cost-effective for the family, the health care system and society in general (less than $50k per QALY). Therefore, it is logical for MSD HCT to be covered by health insurance.
PROBLEM
Refusals of coverage for MSD HCT are concerning to SCDAA. Some of the publicly available insurers’ guidelines (REFERENCES below) appear to be based upon older datasets. The success of MSD HCT and a number of other therapies for SCD make the ethical implementation of a randomized trial of MSD HCT vs. a “control group” very difficult, and insurance companies should not expect such a large randomized controlled trial data to emerge. Similarly, federal agencies are no longer writing guidelines for sickle cell disease care, leaving guidelines to the professional societies like the American Society of Hematology.
The core mission of SCDAA is to work toward a universal cure for sickle cell disease. MSD HCT is well established as potentially curative therapy (although not universal because of the limitation of finding an MSD). SCDAA Medical Advisory and Research Committee (MARAC) offers to help health insurance payers gain an accurate picture of the stratification of risks and benefits of MSD HCT.
RECOMMENDATIONS
MARAC recommends MSD HCT for sickle cell disease should be covered by health insurance policies. Broad eligibility for MSD HCT would be an indicator of insurance coverage that understands the modern cost-effective management of sickle cell disease. Barriers to eligibility would indicate a low-quality policy that perpetuates health system barriers to care and adds to the health disparities in sickle cell disease.
MARAC recommends that modern datasets and the latest analyses should be used in consideration of insurance coverage policies for sickle cell disease, because HCT procedures and eligibility have moved forward since the first landmark studies and progressed even further in the past 10 years. Outcomes are best for individuals below age 5.
The current evidence base includes:
- American Society of Hematology guidelines (Kanter et al. Blood Adv 2021)
- Cost-effectiveness analysis (Arnold et al. Biol Blood Marrow Transplant. 2015)
- Pediatric Bone Marrow Transplant Consortium late-effects guidelines (Shenoy et al. Biol Blood Marrow
Transplant. 2018). - Large review. (Iqbal et al. Transplant Cell Ther. 2021).
- Risk stratification by age. (Brazauskas et al. Blood. 2020).
- Risk stratification by donor and conditioning regimen. (Eapen et al. Lancet Haematol. 2019).
MARAC endorses a patient-centered shared-decision-making process about MSD HCT. The risks and benefits of sickle cell disease are very individualized. However, the insurance payer should not be the gatekeeper for the decision for potentially curative therapy, because cost-effectiveness is already established.
MARAC offers assistance to insurance payers as “content experts” in sickle cell disease. MARAC could provide input on accurate information for insurance policies. MARAC is considering mobilizing a panel of sickle cell experts to provide an evidence-based review of denials of insurance coverage for MSD HCT. We also invite insurers to join the numerous educational sessions conducted by SCDAA and its member organizations.
REFERENCES
Current information about Hematopoietic Stem Cell Transplant for sickle cell disease.
- Kanter J, Liem RI, Bernaudin F, Bolaños-Meade J, Fitzhugh CD, Hankins JS, Murad MH, Panepinto JA, Rondelli D, Shenoy S, Wagner J, Walters MC, Woolford T, Meerpohl JJ, Tisdale J. American Society of Hematology 2021 guidelines for sickle cell disease: stem cell transplantation. Blood Adv. 2021 Sep 28;5(18):3668-3689. doi: 10.1182/bloodadvances.2021004394C. PMID: 34581773; PMCID: PMC8945587
- Cost-effectiveness analysis Arnold SD, Jin Z, Sands S, Bhatia M, Kung AL, Satwani P. Allogeneic Hematopoietic Cell Transplantation for Children with Sickle Cell Disease Is Beneficial and Cost- Effective: A Single-Center Analysis. Biol Blood Marrow Transplant. 2015 Jul;21(7):1258-65. doi: 10.1016/j.
bHCT.2015.01.010. Epub 2015 Jan 20. PMID: 25615608; PMCID: PMC5605133. - Pediatric Bone Marrow Transplant Consortium late-effects guidelines Shenoy S, Gaziev J, Angelucci E, King A, Bhatia M, Smith A, Bresters D, Haight AE, Duncan CN, de la Fuente J, Dietz AC, Baker KS, Pulsipher MA, Walters MC. Late Effects Screening Guidelines after Hematopoietic Cell Transplantation (HCT) for Hemoglobinopathy: Consensus Statement from the Second Pediatric Blood and Marrow Transplant Consortium International Conference on Late Effects after Pediatric HCT. Biol Blood Marrow Transplant. 2018 Jul;24(7):1313-1321. doi: 10.1016/bbmt.2018.04.002. Epub 2018 Apr 10. PMID: 29653206.
The latest evidence.
- Large review. Iqbal M, Reljic T, Corbacioglu S, de la Fuente J, Gluckman E, Kumar A, Yassine F, Ayala E, El-Jawahri A, Murthy H, Almohareb F, Hashmi SK, Cappelli B, Alahmari A, Scigliuolo GM, Kassim A, Aljurf M, Kharfan-Dabaja MA. Systematic Review/Meta-Analysis on Efficacy of Allogeneic
Hematopoietic Cell Transplantation in Sickle Cell Disease: An International Effort on Behalf of the Pediatric Diseases Working Party of European Society for Blood and Marrow Transplantation and the Sickle Cell Transplantation International Consortium. Transplant Cell Ther. 2021 Feb;27(2):167.e1-167.e12. doi: 10.1016/j.jtct.2020.10.007. Epub 2020 Dec 10. PMID: 33830027. - Risk stratification by age. Brazauskas R, Scigliuolo GM, Wang HL, Cappelli B, Ruggeri A, Fitzhugh CD, Hankins JS, Kanter J, Meerpohl JJ, Panepinto JA, Rondelli D, Shenoy S, Walters MC, Wagner JE, Tisdale JF, Gluckman E, Eapen M. Risk score to predict event-free survival after hematopoietic cell transplant for sickle cell disease. Blood. 2020 Jul 30;136(5):623-626. Doi: 10.1182/blood.2020005687. PMID: 32518950; PMCID: PMC7393258.
- Risk stratification by donor and conditioning regimen. Eapen M, Brazauskas R, Walters MC, Bernaudin F, Bo-Subait K, Fitzhugh CD, Hankins JS, Kanter J, Meerpohl JJ, Bolaños-Meade J, Panepinto JA, Rondelli D, Shenoy S, Williamson J, Woolford TL, Gluckman E, Wagner JE, Tisdale JF. Effect of donor
type and conditioning regimen intensity on allogeneic transplantation outcomes in patients with sickle cell disease: a retrospective multicentre, cohort study. Lancet Haematol. 2019 Nov;6(11):e585-e596. doi: 10.1016/S2352-3026(19)30154-1. Epub 2019 Sep 5. PMID: 31495699; PMCID: PMC6813907.
Insurance Coverage Policies about transplant for hemoglobinopathies and/or genetic diseases
- AmeriGroup (November 2021) https://medpol.providers.amerigroup.com/docs/gpp/AGP_HistoricalMedPol_TRANS_00029.pdf?v=202301052014 Accessed 9/15/2023
- NC Medicaid. (August 2023) https://medicaid.ncdhhs.gov/11a-5-allogeneic-hematopoietic-transplant-genetic-diseases-and-acquired-anemias/download?attachment Accessed 9/15/2023
- Premera Blue Cross (Jan 2023) https://www.premera.com/medicalpolicies/8.01.538.pdf Accessed 9/15/2023